Single Particle Li-Ion Battery Model (SPM)
An interactive form of the model is available here
The Single Particle Model (SPM) is a relatively sophisticated physics-based lithium ion battery model.
It incorporates Fick's Law of Diffusion and Butler-Volmer kinetics, but does not include any electrolyte effects,
electrode depth effects, or electrode porosity effects, which can limit the applicability in real-world scenarios.
Specifically, it is at its most valid during low charge / discharge rates.
Below is a representation of a typical cell - it has a cathode, a separator, and an anode. The cathode and anode regions are the ones relevant to this model, which are where lithium is accrued during discharge and charge, respectively.
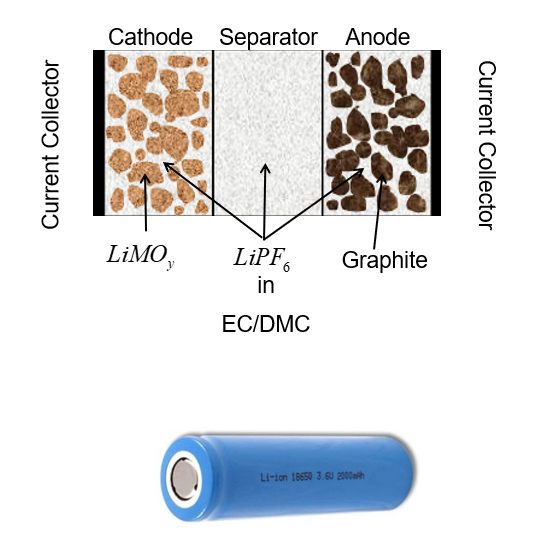
In practical terms, during charge, a lithium ion de-intercalates from the cathode and, upon leaving the surface of the positive electrode particle, is transported to an infinite pool of electrolyte, from where it intercalates into the negative electrode particle and is stored internally. The concentrations in both the positive and negative electrodes are tracked and the surface area is calculated based upon the assumed particle radius, electrode thickness, and active area per unit volume. The particles are all assumed to be perfect spheres of uniform size, meaning the entire electrode can be represented as a single particle, where the current density at the surface is simply scaled to the expected area of an entire cell.

The model can be split into a few different mechanisms:
Mass Transport - Fick's 2nd Law of Diffusion
where concentration has units
and Solid-Phase Diffusion rate is has units
and particle radius has units
This equation describes the way that lithium ions diffuse once inside the particles. This is a partial
differential equation - the left hand side is the derivative with respect to time,
and the right hand side is the derivative with respect to space.
This particular formulation captures the spherical nature of the particles.
For this set of equations, which exist in both the positive and negative particle, we need one initial condition
for time and two boundary conditions for space. The initial condition is the initial concentration at all r, which
is set independently for positive and negative particles:
The boundary conditions are Radial Symmetry (flux = 0) at the center and the ions either enter or leave the surface based on the current :
where is the ionic current, or the rate at which Li ions are entering or leaving the particle, and has units
and is the surface area per unit volume of electrode , which is different for the positive and negative electrodes, and has units
and is the electrode thickness and has units
The current is dictated by Butler-Volmer kinetics, which relate the potential and reaction rate to the current.
This can be thought of as one element of a battery's 'resistance' - in order to move electrons, there has to be a
voltage drop. This really describes how the amount of current that flows relates to the difference between
the external voltage and the battery's Open Circuit Potential (U). The Open Circuit Potential is what gives a
battery discharge curve its shape and really dictates the voltage window of the battery.

Above, the total current is described in terms of the sum of the anode current and the cathode current . This equation captures the nonlinear relationship between a voltage perturbation and the resulting battery current.
where is the anodic or cathodic current density and has units ,
and is the reaction rate for that electrode and has units ,
and is the lithiation fraction of the particle surface and has units ,
and is the solid maximum lithium concentration and has units ,
and is the electrolyte concentration and has units . It is set once and never changes as a core assumption of this model.
For the model (as implemented in Ampere), the following parameters are available:
| name | description | default value | Units |
|---|---|---|---|
| Dn | Li+ Diffusivity in negative particle | 3.9e-14 | m^2/s |
| Dp | Li+ Diffusivity in positive particle | 1e-14 | m^2/s |
| Rn | Negative particle radius | 2e-6 | m |
| Rp | Positive particle radius | 2e-6 | m |
| T | Ambient Temperature | 303.15 | K |
| an | Surface area per unit volume of negative electrode | 723600 | m^2/m^3 |
| ap | Surface area per unit volume of positive electrode | 885000 | m^2/m^3 |
| ce | Starting electrolyte Li+ concentration | 1000 | mol/m^3 |
| csnmax | Maximum Li+ concentration of negative solid | 30555 | mol/m^3 |
| cspmax | Maximum Li+ concentration of positive solid | 51555 | mol/m^3 |
| kn | Negative electrode reaction rate | 5.0307e-9 | m^4/mol s |
| kp | Positive electrode reaction rate | 2.334e-9 | m^4/mol s |
| ln | Negative electrode thickness | 88e-6 | m |
| lp | Positive electrode thickness | 80e-6 | m |